Research Area

Dirty Energy, Smart Technology
Dirty energy smart technology is widely investigated recently, which is converting dirty energy (biomass, coal and tar etc.) to renewable energy by using chemical reaction processes. It is very important to find the way using fossil fuel smartly due to the current sharp increasing trends of oil prices. As one of dirty energy smart technology, KAIST has been studying diesel reforming technology for a solid oxide fuel cell (SOFC) system in 10 years.

Diesel Reforming
Much efforts has been made to feed diesel to the SOFC system because diesel has well constructed infrastructure and high H2 storage density. However, carbon deposition on the reactor was found to be the main barrier from KAIST research and we found that ethylene has critical role in carbon deposition process. We also found the ethylene suppression and cleaning methods and the research has been successful through fuel injector and post-reformer research.

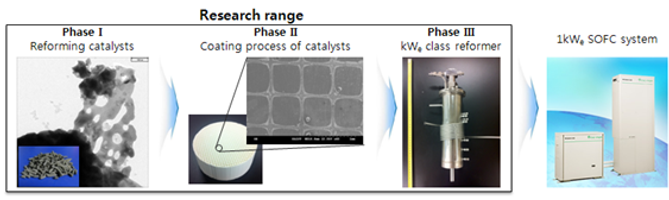
Diesel reforming catalysts are deactivated rapidly during operation because of coke formation, sulfur poisoning and catalysts sintering. Especially, coke formation is severe in diesel reforming because of its high contents of high hydrocarbons. Therefore, inhibition of catalyst deactivations is required to increase reforming efficiency. For this reason, we are researching from reforming catalysts to integrated kW reformer.
SOFC
Solid Oxide Fuel Cells are attractive energy conversion devices for conversion of chemical fuels directly into heat and electricity. The merits of SOFCS are high electric efficiency, low emission of pollutants and fuelflexibility due to high operation temperature. Howver, the high operation temperature, which gives the merits for SOFCs, also is the cause of problems such as limitation selection of materials, thermal degradation of SOFC cell and stack components and oxidation of metal interconnect. These problems can be solved reducing SOFCs operation temperature. Therefore, we are researching new materials for reducing temperature.


SOFCs can use various fuels like methane, natural gas and biogas. The popular anode material of SOFCs is Ni-YSZ (yttrium-stabilized zirconia) cermet. One of the major challenges in SOFC anode is sulfur poisoning problem. When use the fuels including sulfur-containing compounds like hydrogen sulfide, performance of the Ni-YSZ cermet anode is degraded by sulfur poisoning. In addition, Ni-YSZ cermet anode is damaged when oxidation and reduction cycle is repeated. To prevent sulfur poisoning and redox problem and, many researchers make an effort to develop sulfur-tolerant and redox-stable anode materials. So, we are researching oxide catalysts for using anode materials.

One of disadvantages of SOFCs is their mechanical strength. Ceramic supported SOFCs are weak and they can be failed by mechanical load. It can be supplemented by applying metal support. The sealing issue is another disadvantage of ceramic support SOFCs, but it can be solved easily using metal support by welding its boundary. Therefore, it minimizes sealants usage in SOFC stack fabrication, and this allows SOFCs to be applied to various applications including transportation facilities such as vessels and automobiles.

Computational Fluid Dynamics
The heat and mass transfer characteristics of solid oxide fuel cells (SOFCs) need to be considered when designing SOFCs because they heavily influence the performance and durability of the fuel cells. The governing equations, the chemical reaction models, the electrochemical reaction models and the physical property models were calculated simultaneously in SOFC numerical analysis. The current density voltage (IV) curves measured experimentally from a single cell were compared with the simulation data for code validation purposes.

Reformed liquid hydrocarbon fuels provide high energy densities for mobile power applications, such as solid-oxide fuel cells in auxiliary power units. Mixture preparation prior to reaction on a reforming catalyst is certainly one important challenge, as incomplete mixing with air can cause unacceptable temperature overshoots in the catalyst due to overoxidation, and/or deleterious deposit formation. There is a high potential for ethylene pro-duction from gas-phase reactions in the mixing region: ethylene is known as a potential precursor. Therefore, a coupled transport-kinetics model was developed and simulated in this work to analyze the phenomena in the mixing region.

